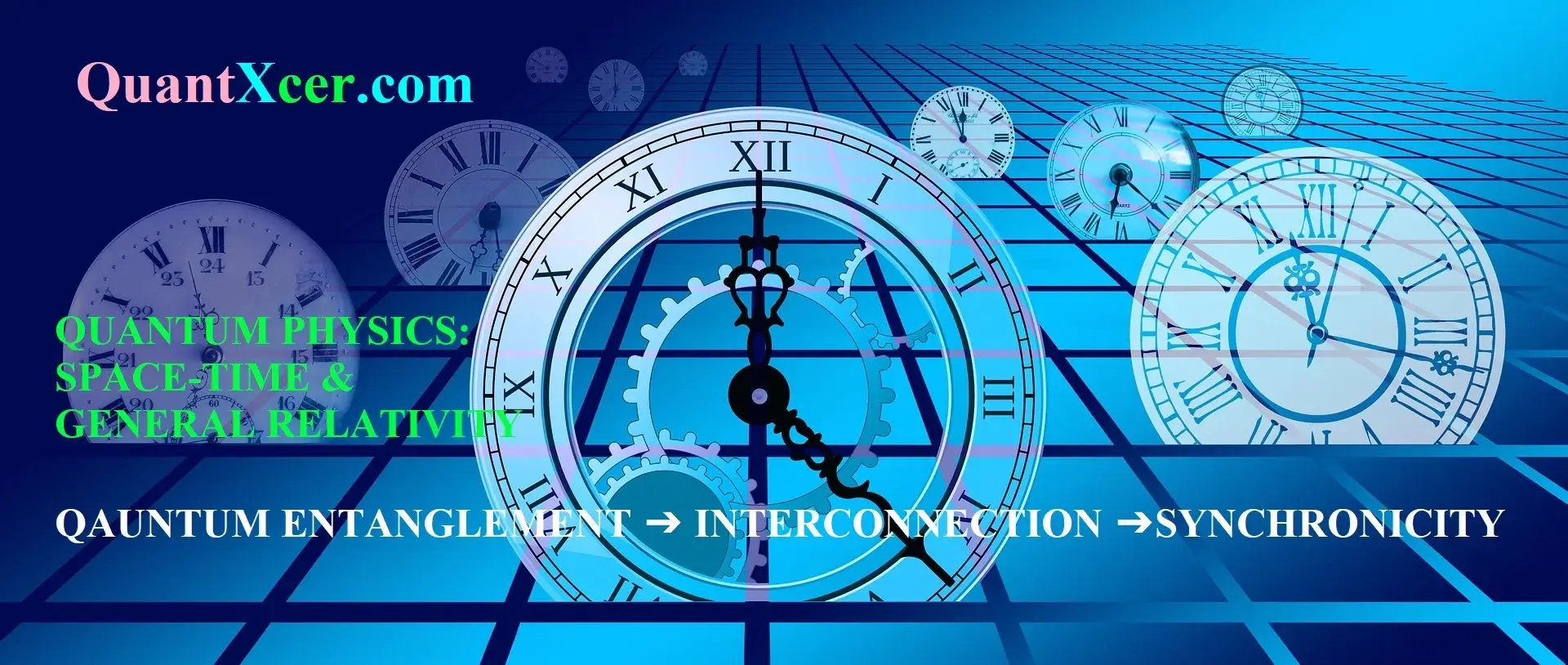
Nuclear Fission
In Figure \(\PageIndex{1}\) we have color coded the nuclei of a given mass \(A=N+Z\) by their mass, red for those of lowest mass through to magenta for those of highest mass. We can see that typically the nuclei that are most stable for fixed \(A\) have more neutrons than protons, more so for large \(A\) increases than for low \(A\). This is the “neutron excess”.
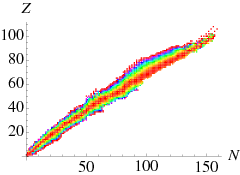
\(\beta\) decay
If we look at the mass of nuclides with fixed nucleon number \(A\) (i.e., roughly perpendicular cuts through the valley of stability in Figure \(\PageIndex{1}\)), we can see that the masses vary strongly,
It is known that a free neutron is not a stable particle, it actually decays by emission of an electron and an antineutrino,
\[n \rightarrow p + e^-+\bar{\nu}_e.\]
The reason that this reaction can take place is that it is endothermic,
\[m_n c^2 > m_p c^2 + m_e c^2.\]
Here we assume that the neutrino has no mass.
The degree of allowance of such a reaction is usually expressed in a \(Q\) value, the amount of energy released in such a reaction,
\[ \begin{align} Q &=m_n c^2 - m_p c^2 - m_e c^2 \\[5pt] &=939.6-938.3-0.5=0.8\text{ MeV}. \end{align}\]
Generically it is found that two reaction may take place, depending on the balance of masses. Either a neutron “\(\beta\) decays” as sketched above, or we have the inverse reaction
\[p \rightarrow n + e^++{\nu}_e.\]
For historical reason the electron or positron emitted in such a process is called a \(\beta\) particle. Thus in \(\beta^-\) decay of a nucleus, a nucleus of \(Z\) protons and \(N\) neutrons turns into one of \(Z+1\) protons and \(N-1\) neutrons (moving towards the right in Figure \(\PageIndex{2A}\). In \(\beta^+\) decay the nucleus moves to the left. Since in that figure I am using atomic masses, the \(Q\) factor is
\[\begin{aligned} Q_{\beta^-}&= M(A,Z)c^2-M(A,Z+1)c^2,\nonumber\\ Q_{\beta^-}&= M(A,Z)c^2-M(A,Z-1)c^2-2m_e c^2.\end{aligned}\]
The double electron mass contribution in this last equation because the atom looses one electron, as well as emits a positron with has the same mass as the electron.
In similar ways we can study the fact whether reactions where a single nucleon (neutron or proton) is emitted, as well as those where more complicated objects, such as Helium nuclei (\(\alpha\) particles) are emitted. I shall return to such processed later, but let us note the \(Q\) values,
\[\begin{aligned} \text{neutron emission} && Q&=[M(A,Z)-M(A-1,Z)-m_n]c^2, \nonumber\\ \text{proton emission} && Q&=[M(A,Z)-M(A-1,Z-1)-M(1,1)]c^2, \nonumber\\ \text{$\alpha$ emission} && Q&=[M(A,Z)-M(A-4,Z-2)-M(4,2)]c^2, \nonumber\\ \text{break up} && Q&=[M(A,Z)-M(A-A_1,Z-Z_1)-M(A_1,Z_1)]c^2.\end{aligned}\]