➤ Blackbody Radiation : Light Manifesting As Both Waves And Particles
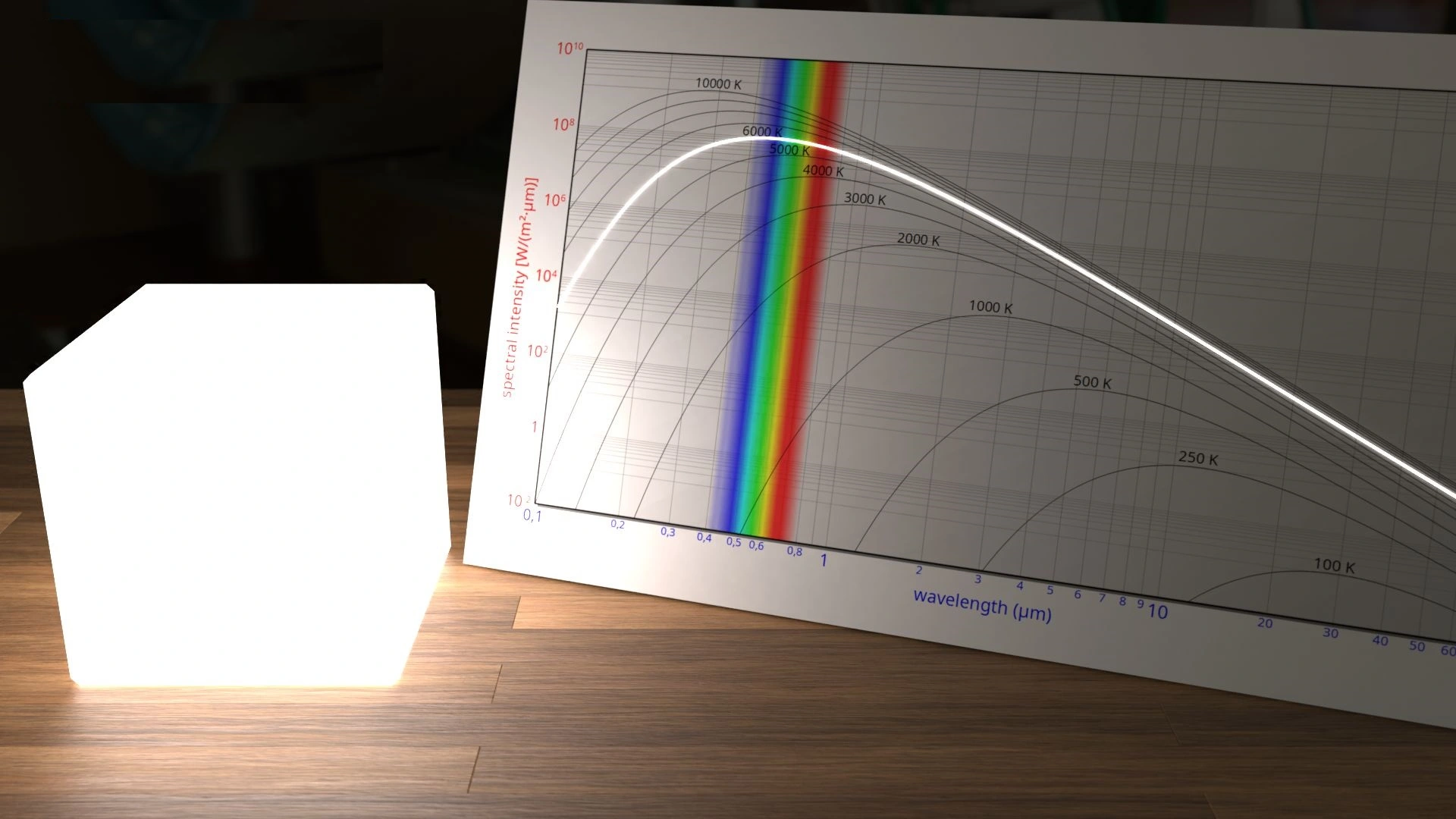
Blackbody radiation is nothing but the phenomena where the surface absorbs all the electromagnetic radiation (all the light) falling upon it.
The fact that light can behave like particles is proved experimentally by examining the radiation spectrum of light emitted by every object.
In addition to proving the particle nature of light, the investigation on the blackbody radiation gave rise to the field of Quantum Physics.
Every object above the absolute temperature of zero (0°K) emits radiation in the infrared range.
The spectrum of that radiated light was studied by Physicists and they found it to be varying based on the temperature of the object.
A perfect blackbody is any object that emits all the light that is incident on it from the environment around it.
Read more...➔
➤Deducing The Equations For The Discrete Energy Levels And Radii Of Electrons In The Atoms
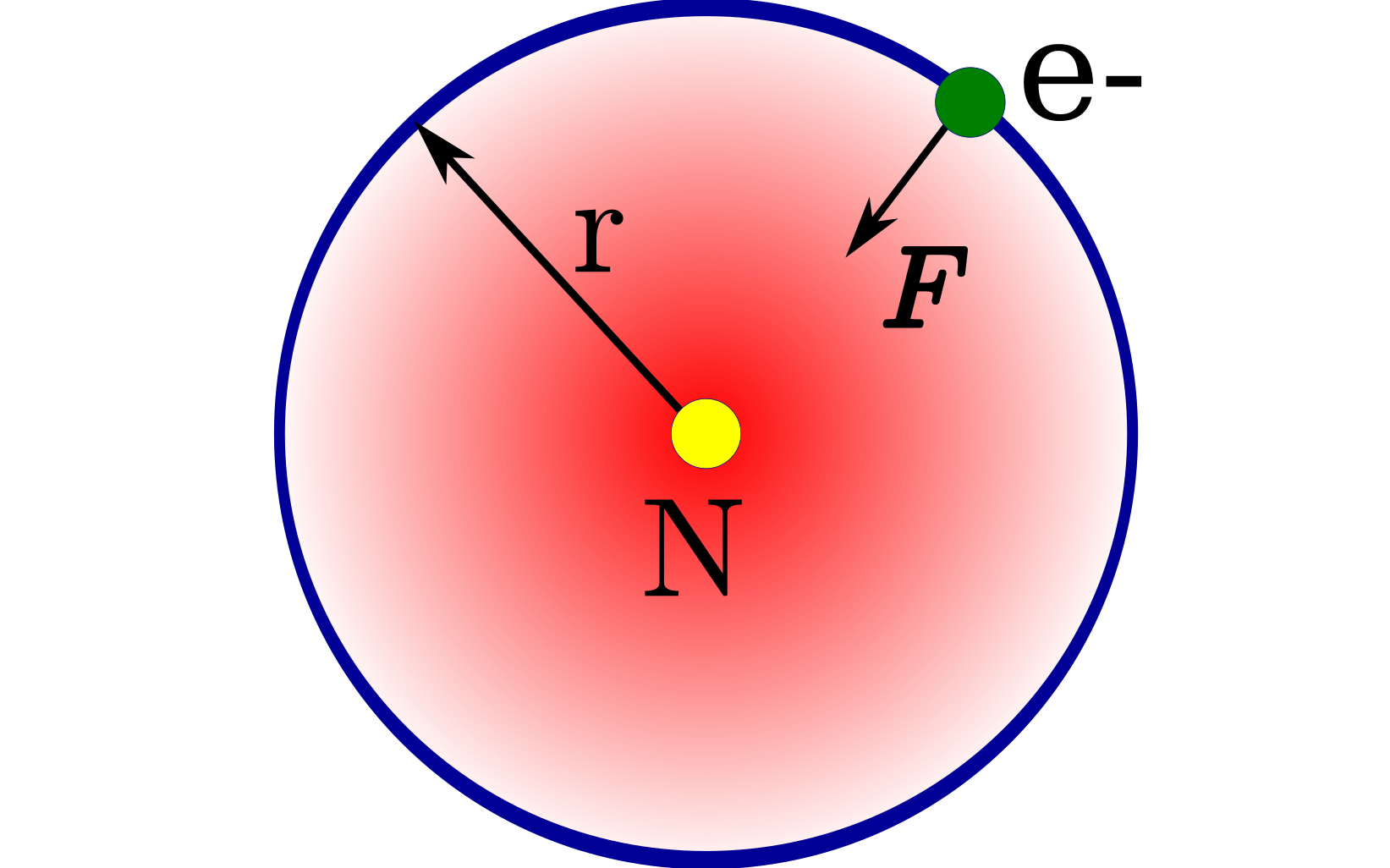
Niels Bohr took to the task of explaining the observed line spectra of one-electron atoms like H,He+,Li2+. He devised a new model for the Hydrozen atom. Combining
the new ideas of Max Planck and Albert Einstein, he hypothesized the new model of the atom. He proposed that electrons can exist only with certain energy levels (or degrees) in the atoms.
They can not have any random energy level. This idea is similar to the theories proposed for the quantization of black body radiation.
Read more...➔
➤ Hydrozen Emission Spectral Lines & Derivation Of Rydberg Constant
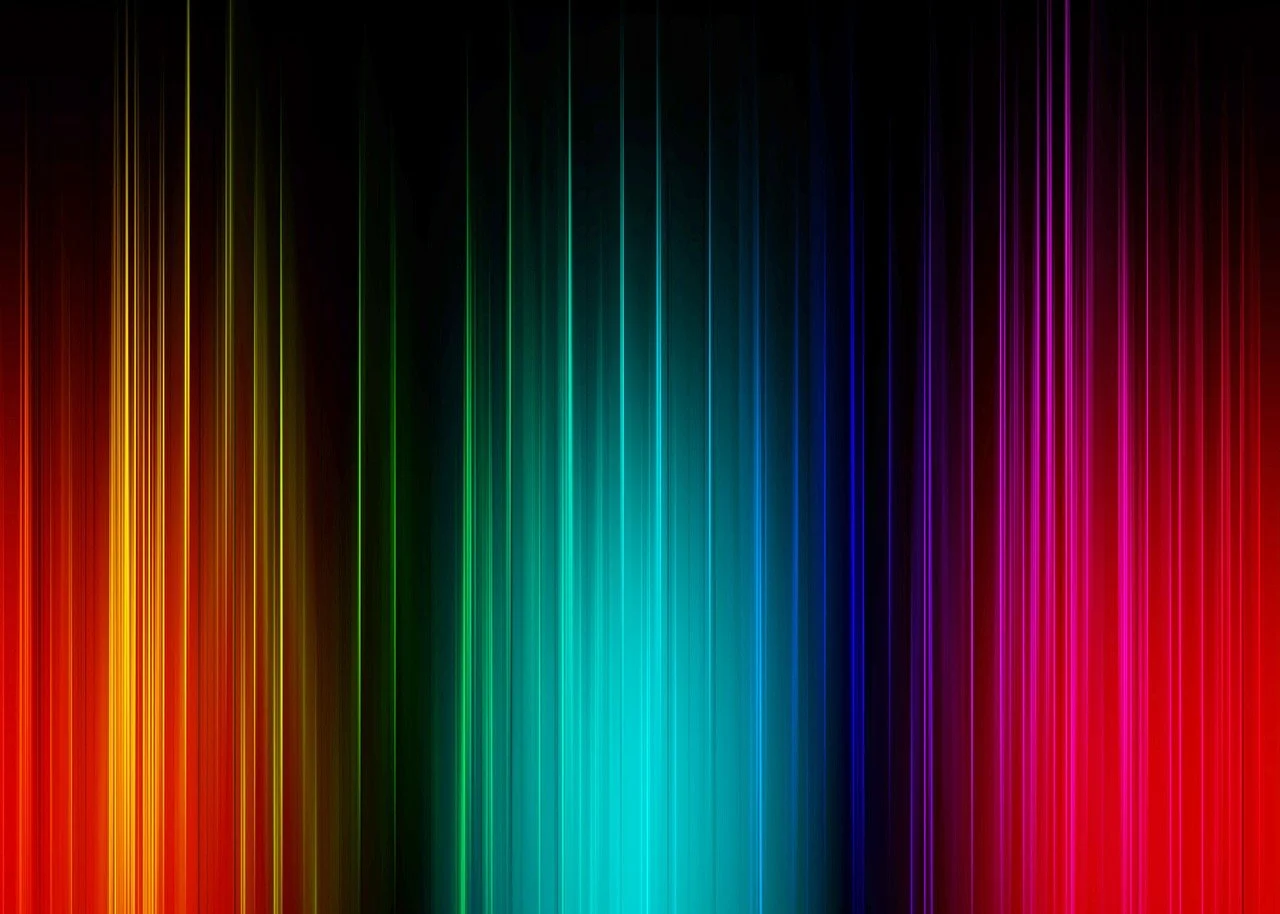
It is a well-known fact that when electrons in an atom or a molecule take in energy and become stimulated (or excited), they transit from a lower energy orbit to a higher energy orbit.
And when they recoil back to the original lower energy state, they emit electromagnetic radiation in the form of photons. This physical process of absorption and emission of photons
explains about the emission spectrum of Hydrozen.
The spectrum seen through a diffraction grating when a gas is stimulated by an electric discharge is made up of distinct,
well-defined wavelengths rather than a continuous band of light.
According to experiments, the wavelengths of the lines were typical of the chemical element that was generating the light.
They were atomic fingerprints that came from the intrinsic structure of the atom.
Read more...➔
➤ Schrödinger Equation Derivation & Its Significance

The classical wave equation is:
\( y(x,t) = A cos(kx-ωt) \)
The above equation represents a simple progressive wave which is travelling towards the right where A is the amplitude, \( k (the \;wave \;number) = \cfrac{2π}{λ} \),
and angular frequency \( ω = 2πf \) where \( f \) is the frequency of the oscillation of the wave, \(x\) is the displacement, \(t\) is the time.
This displacement function is equivalent to the general wave equation:
Read more...➔
Are you a technological scientist?
Do you want to send us your hypothesis composition or
a piece of writing?
Go to:
→Submissions
Subscription For News Letter
High-Energy Physics
Nuclear Physics
QuantumElectroDynamics
QuantumChromoDynamics
Quantum Gravity
Quantum Optics
Quantum Computing
Quantum Consciousness
Quantum Biology
Research Papers
Documentaries
E-Books
About Us
Contact Us
Researches
GDPR Privacy Policy
CCPA Privacy Policy
Editorial Policy
Website Accessibility
Terms of use
PRESS & PUBLIC RELATIONS
Pavan Modi
QuantXcer
Hyderabad, India
Email: pavan.modi.mrk@quantxcer.com